Earth’s Atmosphere Demonstrates Stunning Biocentric Fine-Tuning
Summary: Our planet’s atmosphere provides a beautiful example of the parsimony and elegance of nature’s fine-tuning for aerobic life. This extraordinarily improbable degree of environmental fitness was woven in the order of things.
Editor’s note: Biologist Michael Denton’s new book, The Miracle of Man: The Fine-Tuning of Nature for Human Existence, is out now. This essay is adapted from Chapter 3, “Fitness for Aerobic Life.”
In my book The Miracle of Man, I review a stunning range of coincidences in nature that should inspire both awe and wonder. One striking illustration may be found in our planet’s atmosphere, which provides compelling evidence of a very special fitness for the generation of oxygen for oxygen-hungry beings such as ourselves.
For photosynthesis to proceed on a planet like Earth, sunlight (visual light) must penetrate the atmosphere all the way to the ground, and part of the sun’s infrared radiation needs to be absorbed by the atmosphere so as to warm the planet into the ambient temperature range, where the chemistry of life, including the chemistry of photosynthesis, can work its magic.
Happily, our atmosphere obliges. Earth’s atmosphere absorbs a significant fraction of the infrared radiation — warming the atmosphere into the ambient range — and lets through nearly all of the radiation in the visual region to empower the process of photosynthesis.
Some infrared radiation does reach the Earth’s surface, felt as warmth on the skin, and some penetrates a little way into water, as is commonly experienced in a swimming pool. But there are several major atmospheric absorption bands in the near infrared region that capture and retain the sun’s heat, raising our planet’s surface temperature by 33°C over what it would be without them, a chilly −18°C.
If our atmosphere didn’t absorb at least a significant fraction of the infrared radiation when the sun was shining, the atmosphere would be intolerably hot during the day, and when night fell the temperature would plunge below zero. We would experience wild temperature swings like those on the moon. There temperatures spike in the daytime to more than 100°C (the boiling point of water at sea level) and plunge to −178°C at night, a temperature far, far colder than any experienced on Earth today. This wide variation is because the moon has no atmosphere to retain heat at night or prevent the surface from getting so hot during the day. No type of carbon-based plant life based in a water matrix could survive such massive temperature fluctuations.
On the other hand, if our atmosphere absorbed too much in the infrared region, that too would be disastrous. And this highlights another intriguing element of fitness in the absorption pattern of electromagnetic radiation in the infrared region. The windows between the absorption peaks are as crucial as the peaks. Why? Because without some spectral windows, all the infrared radiation would be absorbed by the atmosphere, none could be radiated back out into space, and Earth would suffer a runaway greenhouse effect, ending up a hellish hothouse like Venus.
A Sizable Absorption Window
In this context an intriguing feature of our atmosphere’s absorption spectrum is a sizable absorption window between eight and fourteen microns. It’s intriguing because the sun is not the only body that emits infrared radiation. The Earth also does, since all bodies at a given temperature emit radiation with a characteristic range of wavelengths. In Earth’s case, the emission peak is in the infrared region near 10 microns. And our atmosphere’s absorption gap allows a significant fraction of Earth’s infrared emission to escape into space through the eight-to-fourteen micron window. Around a fourth of the outgoing infrared emission from Earth escapes through this window, which consequently plays a major role in preventing our planet from going the way of Venus. If all radiation in the infrared between 0.80 and 100 microns had been absorbed by the atmospheric gases, if there were no windows, a runaway greenhouse would have been inevitable. The Earth would be a hot, Venus-like planet. Upon these windows, including the eight-to-fourteen-micron window, all advanced life on the surface of the Earth, including of course Homo sapiens, depends.
It is no exaggeration to say even with all the other elements of fitness that make possible our existence, without this eight-to-fourteen micron window — but one small detail in the atmosphere’s overall absorption spectrum — we wouldn’t exist. This represents yet another stunning instance of the biocentric fine tuning of nature.
Some Additional Fortuities
Before turning to the role of specific atmospheric gases in the fine-tuning of our atmosphere for advanced terrestrial life, a few quick notes on some additional fortuities regarding Earth’s relationship to light.
The light that passes through our atmosphere must penetrate water, not just to gift the sun’s energy to aquatic plants but because water is the matrix of life, and to reach the chloroplasts in any green plant, aquatic or terrestrial light must traverse the water in the cell. Again nature obliges as water is transparent to radiation in the visual band as a liquid, as a vapor in the atmosphere, and as ice. If liquid water or water vapor in the atmosphere absorbed visual light — the right light for photosynthesis — then photosynthesis would not be possible, and Earth would be devoid of aerobic life forms.
Also fortuitous is the transparency of our atmosphere to visible light, which made important scientific advances possible, as Carl Sagan underscored in his 1980 book Cosmos. There he asked us to imagine intelligent life evolving on a cloud-covered planet such as Venus. “Would it then invent science?” he asked. “The development of science on Earth was spurred fundamentally by observations of the regularities of the stars and planets. But Venus is completely cloud-covered… nothing of the astronomical universe would be visible if you looked up into the night sky of Venus. Even the sun would be invisible in the daytime; its light would be scattered and diffused over the whole sky — just as scuba divers see only a uniform enveloping radiance beneath the sea.”
Finally, it is not just that our atmosphere lets through the right light. It also strongly absorbs radiation from the dangerous or potentially dangerous regions of the electromagnetic spectrum on either side of the visual and near infrared regions.
The Atmospheric Gases
Another remarkable aspect of the absorption characteristics of Earth’s atmosphere is that it arises from the combined absorption spectra of the atmospheric gases, five of which — nitrogen (N2), oxygen (O2), ozone (O3), carbon dioxide (CO2), and water vapor (H2O) — are bound to be present in the atmosphere of any planet hosting complex carbon-based biological life. It is their combined absorption characteristics which lets through just the right light for photosynthesis while at the same time absorbing just the right amount of heat, as well as most of the harmful radiation outside of the visual and infrared regions.
Oxygen
Oxygen (O2) is indispensable to complex organisms such as ourselves. We need a lot of it (250 ml every minute, even at rest). Indeed, the metabolic rates needed to sustain the most advanced biological life depend on taking oxygen directly from an atmosphere. Atmospheres sustaining complex aerobic life will inevitably contain substantial quantities of oxygen.
Ozone
Where there is O2 in an atmosphere there is bound also to be ozone (O3), since it’s formed in the stratosphere by the reaction of individual oxygen atoms with molecules of dioxygen, catalyzed by the action of UV light.
O2 + O = O3
Ozone is important to life because it absorbs harmful ultraviolet radiation.
Carbon Dioxide
Breathing involves taking in oxygen and exhaling carbon dioxide (CO2), which is a major product of aerobic metabolism (the process which provides us with 90 percent of our energy needs). Consequently CO2 will be found in the atmosphere of any planet where organisms use the oxidation of reduced carbon to generate energy. Carbon dioxide is also essential to plants, which require it for photosynthesis. Moreover, CO2 is the only feasible carrier of the carbon atom to all parts of any carbon-based biosphere.
CO2 is also delivered to the atmosphere on Earth by volcanic activity and is recycled via silicate weathering.
Water Vapor
Atmospheric water vapor will be found in the atmosphere of any planet harboring abundant carbon-based life because water is the essential physical matrix of all carbon-based cells and it is the necessary medium of the circulatory system in all complex multicellular organisms. Only worlds that possess water can harbor carbon-based life, hence the NASA adage “follow the water” in searching for extraterrestrial life. And since water evaporates at temperatures fit for biochemistry, some water vapor is bound to be present in the atmosphere of any world bearing carbon-based life.
Nitrogen
Atmospheric nitrogen provides most of the nitrogen atoms incorporated into organic compounds by life on Earth. It’s one of the four core atoms of organic chemistry alongside carbon, oxygen, and hydrogen. It provides necessary density to the atmosphere, keeps our oceans from evaporating, and serves as a fire retardant, slowing the speed that fire spreads, rendering it controllable. Nitrogen is the only viable candidate for these roles and thus appears to be an essential ingredient in the atmosphere of any planet hosting carbon-based life.
All this suggests that oxygen, nitrogen, water vapor, carbon dioxide, as well as ozone are bound to be present in the atmosphere of any world inhabited by oxygen-utilizing, advanced carbon-based life, for reasons over and above their life-friendly atmospheric transparency for the right kinds of electromagnetic radiation.
The Right Proportions
Our atmosphere not only has the right components for complex aerobic life, it also has them in the right proportions. Only an oxygen concentration of about 20 percent, at a partial pressure of more than 80 mm Hg, provides the requisite oxygen for the active metabolism of organisms like ourselves. If the concentration were substantially higher, fires would be a far greater danger. In the case of nitrogen, only a considerable quantity of nitrogen provides the density and pressure needed to keep fires from raging uncontrollably in oxygen-rich atmospheres such as Earth’s, and to prevent the oceans from evaporating.
CO2 levels have varied throughout geological time, although over the past 400 million years — since advanced life colonized the land — they have almost certainly never reached levels ten times those of today and probably never more than about four to five times present levels. A recent study provided evidence of this. It found that raising CO2 levels in controlled atmospheres up to four times present levels diminished cognitive function in human subjects. This gives some indication of a CO2 ceiling, beyond which advanced life may no longer viable.
The Greenhouse Gases
Diatomic molecules with the same two atoms, such as O2 or N2, do not absorb infrared radiation. This is quite fortunate for life on Earth, since if either of these two gases, which make up most of our atmosphere, were strong absorbers of infrared radiation, Earth likely would have become a boiling cauldron like Venus, with temperatures hot enough to melt lead.
Also fortuitous: the major greenhouse gases CO2 and H2O are both stable in the presence of O2. This is enormously important. If they were unstable in the presence of oxygen, the whole atmospheric system and global heat balance would collapse. Aerobic life, our sort of life, would be impossible. However, in keeping with nature’s profound fitness for advanced life as it exists on Earth, H2O and CO2 are fully oxidized and stable in the presence of oxygen. Nitrogen, the major component of the atmosphere, is also stable in the presence of oxygen, because the nitrogen atoms in N2 bond strongly with each other and resist combining with oxygen. The stability of water, carbon dioxide, and nitrogen in the presence of oxygen is a point worth underscoring, since most other substances (apart from the noble gases) react strongly with oxygen — in some cases, explosively.
A fascinating further teleological aspect to all this concerns the quantity of ozone in the atmosphere. Because of the vast amounts of O2 in the atmosphere, inevitably there will also be some ozone (O3). Although ozone is indispensable for blocking harmful ultraviolet radiation, it is also a powerful greenhouse gas that absorbs strongly in the infrared region — one thousand times more strongly than CO2. Because of this, anything beyond trace amounts of ozone would contribute dangerously to the greenhouse effect. This means that its life-giving fitness in absorbing the dangerous ultraviolet radiation between 0.20 and 0.30 microns would be negated entirely if more than trace amounts were necessary for that vital task, or if it were produced in excess amounts by the action of ultraviolet radiation on O2 in the stratosphere. Happily, only trace amounts are needed to effectively block harmful ultraviolet radiation, and the rate of breakdown of ozone in the stratosphere almost equals its rate of synthesis, guaranteeing that it is indeed only present in trace amounts.
Finally, an intriguing aspect of ozone’s synthesis in the atmosphere is that ozone (O3) and diatomic oxygen (O2) indirectly promote their own formation by absorbing dangerous ultraviolet radiation and thereby protecting plant life, both aquatic and terrestrial, which synthesize the oxygen from which ozone is formed. This is yet another beautiful example of the parsimony and elegance of nature’s stunning fitness for aerobic life.
Vital Coincidences
The absorption properties of our atmosphere are not vital for all carbon-based life on Earth, but particularly for plants and energy-hungry aerobes like ourselves. Our atmosphere’s fortuitous mix of gases enables photosynthesis and the manufacture of oxygen, warms Earth into the ambient temperature range, and shields life from harmful radiation. Even slight differences in our atmospheric gases’ absorption properties, or in their relative concentration, and Earth would be uninhabitable, particularly for aerobic life. And note, these gases exist in our atmosphere, and in the proportions they do, because of factors quite distinct from the life-essential absorption properties described above.
There is a final twist to this teleology: Three of the key atmospheric gases whose physical absorption properties are indispensable to the process of photosynthesis are also central players in the process of photosynthesis itself.
6CO2 + 6H2O +light +heat —> C6H12O6 + 6O2
Indeed, they are the major reactants in the process. It is as if CO2, H2O, and O2 were deliberately colluding to incorporate themselves into the stuff of living matter.
Light and Air
Let’s review. The laws of nature, which determine the absorption properties of the atmospheric gases, have no logically necessary connection with their chemical properties or the chemical properties of their constituent atoms, which are of such utility to life. This is a striking fortuity in the nature of things.
Similarly, there is no connection between the laws of nature which determine the tiny size of the biologically useful region in the electromagnetic spectrum, and those laws which determine the radiant output of the sun. And there is no connection between the radiant output of the sun and the laws determining the absorption properties of the atmospheric gases and liquid water.
So here we have several coincidences on which the existence of oxygen-hungry aerobic organisms like ourselves depends. In the 15th edition of the Encyclopaedia Britannica, in the article entitled “Electromagnetic Radiation,” the authors comment, “Considering the importance of visible sunlight for all aspects of terrestrial life, one cannot help being awed by the dramatically narrow window in the atmospheric absorption… and in the absorption spectrum of water.”
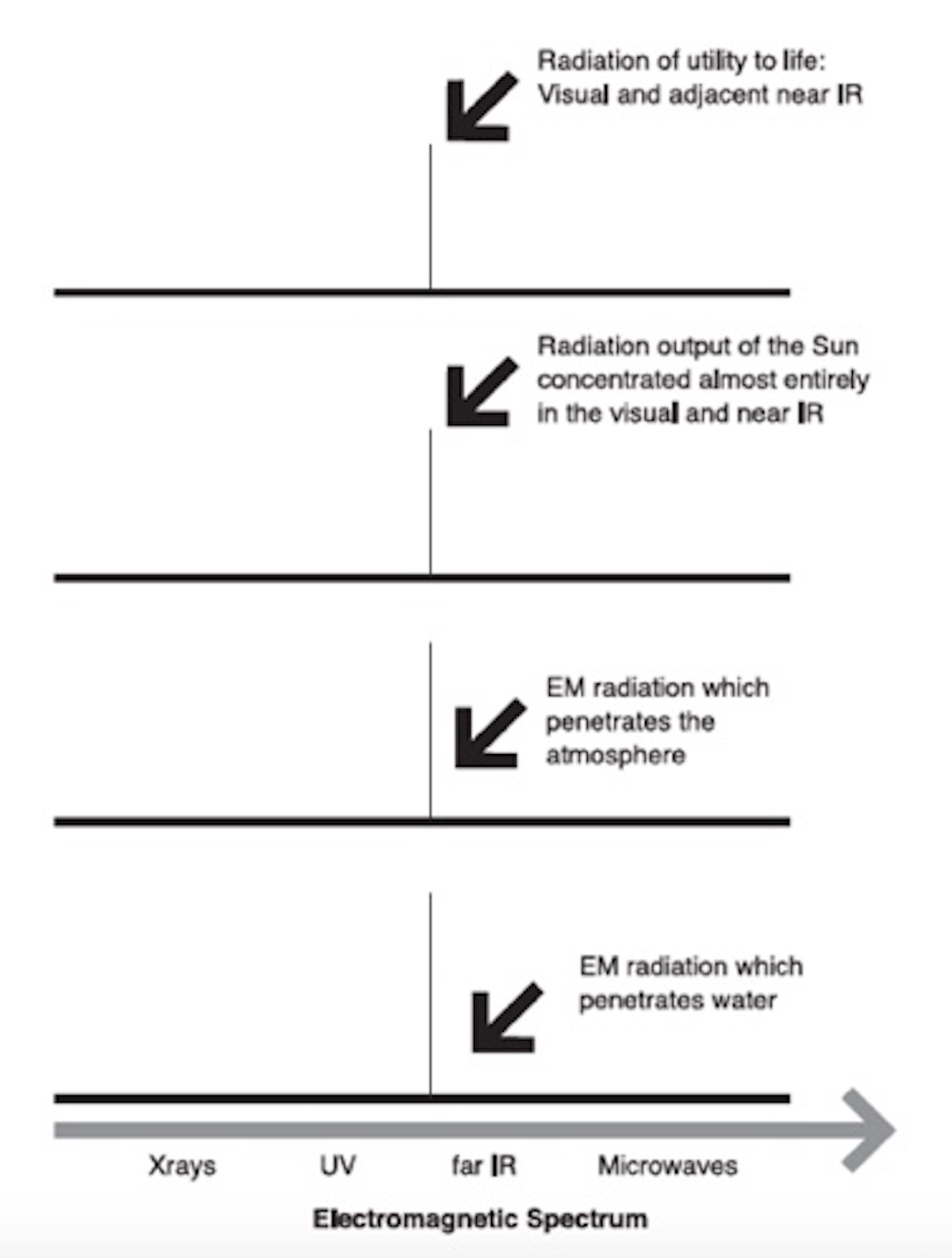
And it isn’t just the “dramatically narrow window.” We should be in awe of the entire ensemble of prior environmental fitness, an ensemble that enables photosynthesis and, by extension, our own existence as oxygen-hungry “light-eaters.”
Simply put, our existence, inhabiting the surface of a planet like Earth, deriving energy generated by the oxidation of the reduced carbon compounds manufactured during the process of photosynthesis, depends on what can only be described as an extraordinarily improbable degree of environmental fitness in the order of things. Note, too, that the improbable coincidences reviewed above, and much more fully treated in The Miracle of Man, are largely irrelevant to the other major domain of carbon-based life on our planet — the great biomass of “rock-eating” anaerobic denizens of the dark. Nature’s awe-inspiring fitness for photosynthesis is a fitness for our type of life, for life in the light, for life on a planetary surface, for creatures such as ourselves.
No comments:
Post a Comment